Dear Partners:
Please review the following recall below, check your inventory for affected products, and take necessary action.
Post this recall notice where those accessing your organization can see it (e.g., bulletin board, website, social media).
If you are not the most appropriate person to receive this notice, please forward this communication to the responsible representative at your organization.
To ensure recalls are sent to the appropriate person in the future, please contact Dare to Care to update the appropriate contact information.
You can find a list of all the current recalls on our website here: Important Information – Dare To Care
Let us know if you have any questions or concerns.
________________________________________________________________________________
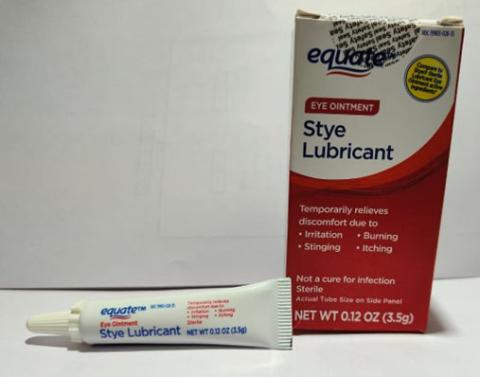
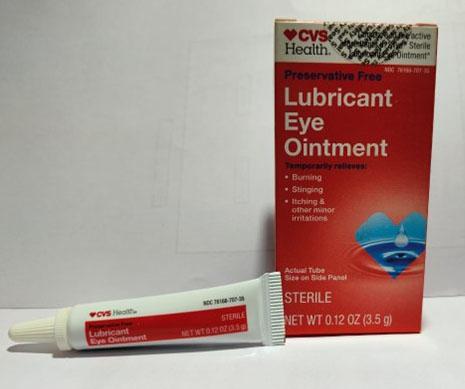
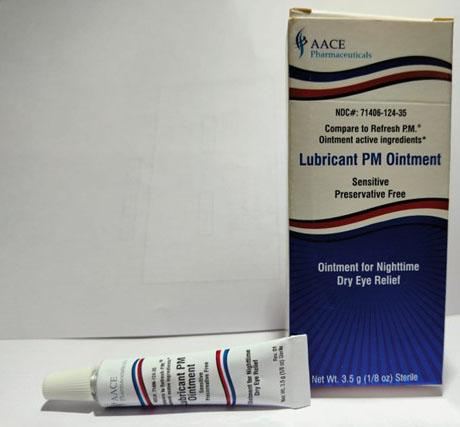
Brassica Pharma Pvt. Ltd. is voluntarily recalling Eye Ointment products listed in the table below with expiration date ranging from February 2024 to September 2025. The products are being recalled due to lack of sterility assurance at the facility noted during an inspection conducted by the Food and Drug Administration (FDA).
Risk Statement: For those patients who use these products, there is a potential risk of eye infections or related harm. These products are intended to be sterile. Ophthalmic drug products pose a potential heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses. To date, Brassica Pharma Pvt. Ltd. has not received any reports of adverse events up to 16th February 2024 related to this recall.
| Product Name | Package Description | Brand Name | NDC | Lot# Exp Date | Exp Date |
| Equate Lubricant Eye Ointment (Mineral Oil 42.5%, White Petrolatum 57.3%, Lanolin Alcohols) | 3.5 gram tube, packaged in cardboard box with UPC Code 681131395298 | Equate | 79903-026-35 | A2E01 | Apr-24 |
| A2L05 | Nov-24 | ||||
| A3B01 | Jan-25 | ||||
| A3C01 | Feb-25 | ||||
| A3H05 | Jul-25 | ||||
| Equate Stye Lubricant Eye Ointment (Mineral Oil 31.9%, White Petrolatum 57.7%, Microcrystalline Wax, Stearic Acid, Wheat Germ Oil) | 3.5 gram tube, packaged in cardboard box with UPC Code 681131395304 | Equate | 79903- 028-35 | A2D08 | Mar-24 |
| A2F02 | May-24 | ||||
| A2I03 | Aug-24 | ||||
| A2L03 | Nov-24 | ||||
| A2L04 | Nov-24 | ||||
| A3C03 | Feb-25 | ||||
| A3C05 | Feb-25 | ||||
| A3H01 | Jul-25 | ||||
| A3H03 | Jul-25 | ||||
| CVS Health Lubricant Eye Ointment (Mineral Oil 31.9%, White Petrolatum 57.7%, Microcrystalline Wax, Stearic Acid Wheat Germ Oil | 3.5 gram tube, packaged in cardboard box with UPC Code 050428634141 | CVS Health | 76168- 707-35 | A2F03 | May-24 |
| A2I02 | Aug-24 | ||||
| A2L02 | Nov-24 | ||||
| A3C04 | Feb-25 | ||||
| A3H04 | Jul-25 | ||||
| Lubricant PM Ointment | 3.5 gram tube, packaged in cardboard box with UPC Code 371406124356 | AACE Pharmaceuticals | 71406- 124-35 | A2G01 | Jun-24 |
| A2G02 | Jun-24 | ||||
| A3F08 | May-25 | ||||
| A3F09 | May-25 | ||||
| A3J17 | Sep-25 | ||||
| A3J18 | Sep-25 |
These products were distributed nationwide to wholesalers, retailers, and via the product distributor, Walmart, CVS and AACE Pharmaceuticals Inc.
Brassica Pharma Pvt. Ltd. Is notifying its distributors AACE Pharmaceuticals Inc and its retailers Walmart and CVS. These distributors shall be further notifying the wholesalers and retailers via mail of this voluntary recall and arranging for return of all impacted products listed above. Consumers, distributors and retailers that have any product which is being recalled should cease distribution of the product. Consumers should stop using the recalled Eye Ointment and may return any of the above listed products to the place of purchase.
Consumers with questions regarding this recall can contact Brassica Pharma Pvt. Ltd. at +1 833-225-9564 or info@brassicapharma.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
Consumers:
Brassica Pharma Pvt. Ltd
+1 833-225-9564